Steps to an Acid/Base Problem:
- Step 1: Check for internal consistency[H+] = 24 x pC02/HCO3
- Step 2: Use pH to determine primary disorder
- Step 3: Calculate the AGAG = [Na+] – [Cl- + HCO3]
- Step 4: Determine the presence of additional disordersCorrected HCO3 = measured HCO3 + [AG – 12]
- Step 5: Calculate the expected pC02 for metabolic acidosis (Winter’s Formula)Expected PC02 = 1.5 (HCO3) +8 +/- 2
5 Etiologies with AST/ALT elevations > 1000:
- Drugs/Toxins (acetaminophen, toxic mushrooms, etc.)
- Hepatic ischemia
- Acute viral hepatitis
- Acute autoimmune hepatitis
- Wilson disease
3 components that define acute liver injury:
- Elevated aminotransferases
- Hepatic encephalopathy
- Elevated PT/INR
*No preexisting cirrhosis or liver disease; <26 weeks
Acetaminophen Toxicity:
- 4 grams is the daily maximum recommended dose
- N-acetylcysteine is the treatment of choice in suspected acetaminophen toxicity -nmay be given IV, using a 20 hour protocol, or orally, using a 72 hour protocol
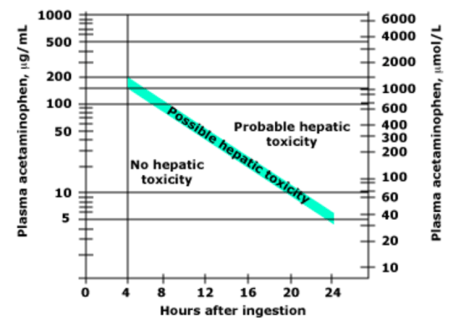
- Use the Rumack-Matthew nomogram to elevate for poisoning severity
Options for renal replacement therapy (RRT):
- Hemodialysis
- Peritoneal dialysis
- Transplantation
- Non-dialytic management – manage symptoms and focus on QOL
Peritoneal dialysis – a dialysate solution is intermittently instilled into the peritoneal cavity via an indwelling catheter, and excess water/solutes are removed by osmosis and diffusion across the peritoneal membrane into the dialysis solution.
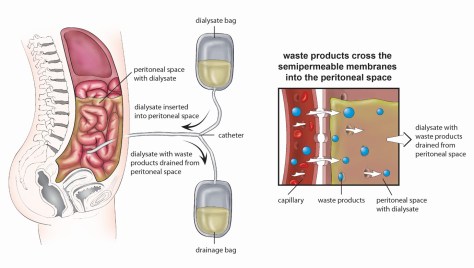
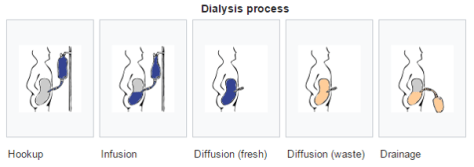
Two different options for PD exist:
- Continuous ambulatory peritoneal dialysis (CAPD)
- Automated peritoneal dialysis (APD)
*CAPD does not require a machine, while APD relies on a “cycler” to fill and empty your belly 3-5 times during the night
*There is no difference in clinical outcome between PD and HD – although there is a trend toward superior outcomes for PD patients with new-onset ESRD who have residual kidney function
Potential complications with PD:
- Peritonitis – from frequent access via the catheter or from perforation or microperforation of bowel
- Hernias at catheter site
- Subcutaneous edema
- Difficulty draining catheter (often due to constipation)
- Pleural effusions
- Sclerosing peritonitis: scarring/fibrosis from recurrent peritonitis – can lead to bowel constriction. Prognosis is extremely poor (often fatal) and treatment requires surgical stripping.
Diagnosis of peritonitis requires 2 out of 3 of the following:
- Clinical signs of peritonitis
- Peritoneal fluid WBC > 100 cells/mm3 with >50% PMNs
- Positive peritoneal fluid culture
Common clinical manifestations of PD related peritonitis:
- Abdominal pain (79-88%)
- Cloudy peritoneal effluent (84%)
- Fever (>37.5 C) (29-53%)
- Nausea/Vomiting (31-51%)
- Hypotension (~18%)
Most common organisms of peritonitis in PD patients:
- Gram-positive organisms ~ 50% -Coagulase-negative staph (S. epidermidis) and S. aureus
- Gram-negative organisms ~ 15%
- Fungal ~2%
- Polymicrobial ~ 4%
- Culture negative ~ 20%
Empiric Antibiotic Regimen:
- Gram positive coverage – typically Vancomycin (or 1st generation cephalosporin)
- Gram negative coverage – typically 3rd generation cephalosporin
- Fungal prophylaxis while on ABX – typically nystatin
Indications for PD catheter removal:
- Relapsing peritonitis – episode of peritonitis with the same organism that caused the preceding episode within 4 weeks of completing antibiotics
- Refractory peritonitis – peritonitis that does not respond to appropriate antibiotics within 5 days
- Refractory catheter infection – exit-site/tunnel infections
- Fungal or mycobacterial peritonitis