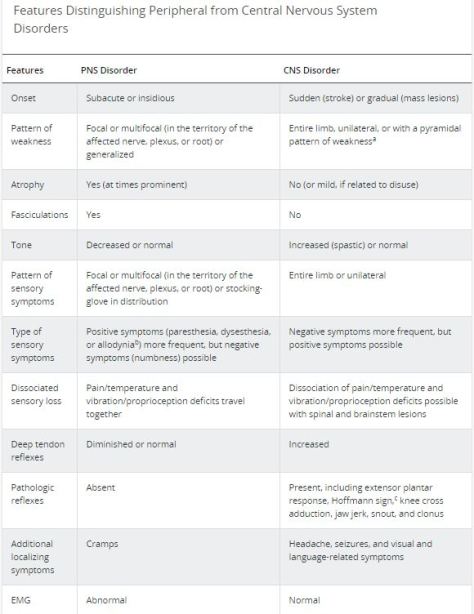
If you see ground-glass opacities on CXR/CT scan and suspect patient has ILD, make sure you consider the differential diagnosis and you look for secondary etiologies!
Limited DDX: atypical PNA, fungal pneumonia (eg: Cocci, Blasto,Histo), CHF
Secondary etiologies (not an exhaustive list)
1)Environmental agents (Asbestos, Beryllium, Silicosis, Pneumoconiosis)
2)Drug induced (long list)-Include Amiodarone, Methotrexate, Bleomycin, Nitrofurantoin , NSAIDS
3)Connective Tissue Disease related-RA. SLE, Scleroderma, MCTD, Sjogrens
4)Radiation
5)Granulomatous diseases (eg: Sarcoid)
6)Vasculitis (eg: Churg Strauss)
7)LAM (lymphangioleiomyomatosis)-Usually young women of child-bearing age with recurrent PTX and chylous effusions, associated with Tuberous Sclerosis
8)Acute eosinophilic PNA-see >25 % eosinophils on BAL
9)Langerhans cell histiocytosis
If no etiology is known, then consider IDIOPATHIC INTERSTITIAL PNEUMONIA
This includes:
- Idiopathic pulmonary fibrosis (IPF, also called Usual Interstitial PNA as the pathologic description)-WORST prognosis, does not respond to steroids, AND diagnosis can be made on HRCT if typical findings, especially if honey-combing (fibrosis)
- Desquamative interstitial PNA (DSIP)- strong association with smoking
- Respiratory bronchiolitis-ILD (RB-ILD)-strong association with smoking
- Non-Specific Interstitial PNA (NSIP)-can be due to HIV, CTD, hypersensitivity reactions or drug related
- Cryptogenic Organizing PNA (COP)-classically presents with patient with persistent symptoms despite multiple antibiotic treatments, responds to steroids
- Acute interstitial PNA (AIP)-very rapid onset, produces ARDS like picture
- Lymphocytic interstitial PNA (LIP)-increased lymphocytes on BAL
S&S:
-Most commonly, progressive DOE and dry cough. Exam reveals crackles (“velcro-like”)
Workup:
-Look for secondary etiologies based on history and drug exposure.
-Check serology for auto-immune disease
-Bronchoscopy, BAL and trans-bronchial biopsy if necessary
-PFT to evaluate for restrictive disease
-VATS (video-associated thoracoscopic surgery) may be necessary if diagnosis is not clear (remember that IPF can sometimes be diagnosed via HRCT and other etiologies may be suggested on imaging)
Remember that IPF has the worst prognosis and does NOT respond to steroids so its important to make that distinction.