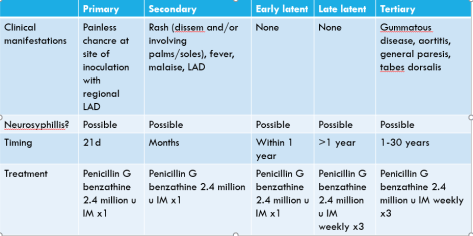
We presented a fascinating case of a middle aged woman who presented with fatigue, weakness, and a significant weight loss in the past year who was found to have hypotension refractory to a usual fluid challenge. Her AM cortisol was low and her post-stim cortisol remained <18, confirming the diagnosis of adrenal insufficiency. Her ACTH was inappropriately on the low end of normal, consistent with a central AI (secondary or tertiary). Here are the highlights:
Clinical Manifestations in Primary AI only:
- Generalized hyperpigmentation of the skin and mucus membranes (exclusively in chronic primary adrenal insufficiency)
- Hyponatremia/hyperkalemia and hypoglycemia generally occurs in primary AI only
Diagnosing Adrenal Insufficiency
- An AM cortisol < 3 is sufficient, but a ACTH stimulation test is preferred
- Check the ACTH level prior to the test!
- After giving the standard-high dose of 250mcg of synthetic ACTH, a cortisol is checked 1 hour later and if <18, confirms the diagnosis of adrenal insufficiency
- If the patient has AI, then look at the ACTH (which will take some time to result anyway)
- If normal (inappropriately so) or low, this is ACTH dependent AI, also known as central AI (in secondary the problem is the pituitary and in tertiary, the problem is in the hypothalamus)
- If high, then this is ACTH-independent AI, also known as primary AI (AKA the problem is with the adrenal gland)
Causes of Primary AI and Secondary AI
- The causes for both are lengthy, but remember the most common etiology for each!
- The most common cause of primary AI in the developed world is autoimmune adrenalitis
- The most common cause of central AI is discontinuation of glucocorticoids