Thank you Charles for presenting this really interesting case. A 18 year old woman with a history of asymptomatic thrombocytopenia who presents with several days of non-specific fever, chills, malaise, mild shortness of breath and she was found to have acute anemia, thrombocytopenia, elevated transaminitis, and patchy bilateral pulmonary infiltrates on CXR during initial presentation. She became acutely ill with submassive hemoptysis and went into respiratory failure in 24-48 hours. She was found to have DAH on BAL. Her autoimmune and infectious work up came back negative, but her ferritin came back at 75776. Base on this and her constellation of symptoms, further work up revealed a 6/8 criteria for diagnosis of hemophagocytic lymphohistiocytosis!
DAH
Presentation
- Dyspnea, cough fever, respiratory failure, acute anemia
- Hemoptysis only in 2/3 of cases
- Definition: Hemoptysis, diffuse alveolar infiltrates, acute anemia, and hypoxemic respiratory failure
Pathophysiology
- Widespread damage to pulmonary small vessels, leading to blood within the alveoli eventually causing impaired gas exchange.
- Causes: Autoimmune/connective tissue disease leading to pulmonary vasculitis (ANCA, anti-GBM), certain pulmonary infections, toxins, drug reactions, mitral stenosis in some cases
- 3 distinct histologic subtypes that can give hints to underlying pathology
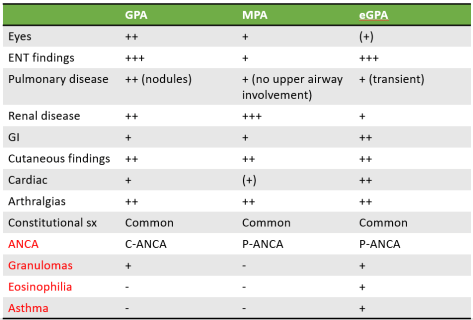
- Most common: Pulmonary capillaritis: ANCA vasculitis, GPA, EPGA, pauci-immune, Goodpasture, HSP, SLE, RA, APLS, MCTD, Behcet, drug-induced, lung transplant rejection, etc.
- Systemic vasculitis manifestation
- Bland pulmonary hemorrhage: Coagulopathy, mitral stenosis, toxin/inhalation, SLE, drugs, Goodpasture
- Anti-GBM, SLE, no inflammation or destruction of capillaries but RBC leakage
- Diffuse alveolar damage: BM transplantation, radiation, ARDS, cytotoxic drugs, other causes
- Most common: Pulmonary capillaritis: ANCA vasculitis, GPA, EPGA, pauci-immune, Goodpasture, HSP, SLE, RA, APLS, MCTD, Behcet, drug-induced, lung transplant rejection, etc.
Diagnosis
- CXR: Diffuse bilateral alveolar infiltrates, no pathognomonic findings
- BAL: serial bloody aspirate with sequential sampling

- CT: Non-specific GGO
- Biopsy: Tissue biopsy of the lung is definitive in confirmation of DAH but underlying cause might not be revealed.
Management
- Treat underlying cause
- Respiratory support, most patients die from respiratory failure
- High dose corticosteroids, i.e. methylprednisolone up to 500mg Q6H (up to 2g daily)
- Other agents: Cyclophosphamide, azathioprine, MTX, mycophenolate, etanercept.
- Plasmapheresis for Goodpasture or vasculitidies.
- Key: Early identification and treatment
HLH
Epidemiology
- Worldwide incidence is unknown, not enough data available, thought to be rare AND underrecognized but growing recognitive leads to higher incidence.
- Familial types: more common to occur in pts < 18yo
- Secondary HLH: any age
Pathophysiology
- Uncontrolled hyperinflammatory response with dysregulated macrophage activity leading to excessive cytokine production
- Primary: HLH due to an underlying genetic abnormality or without clear cause
- Autosomal recessive familial HLH
- Idiopathic
- Secondary: Due to something else
- Retrospective study at Mayo in 2014:
- Infection (34%), most commonly EBV
- Autoimmune (8%), Macrophage activation syndrome (MAS), most often associated with AOSD, systemic juvenile idiopathic arthritis, or SLE.
- Malignancy (52%) NHL, HL, acute leukemia
- Idiopathic/Immune deficiency/other (6%)
- Retrospective study at Mayo in 2014:
Presentation
- Fever, splenomegaly, cytopenias are most common
- + manifestation of the trigger
- Complications: Infection, DIC, bleeding complications (reports of intracranial hemorrhage, GIB, DAH), end organ damage.
Diagnosis: Per the Histiocyte Society: 5/8 criteria for diagnosis. In case you cannot remember all 8, please refer here for the famous HLH Song by Dr. Eric Lau:
-
- Fever
- Splenomegaly
- Peripheral cytopenia (> 2 cell lines)
- Hypertriglyceridemia or Hypofibrinogenemia
- Elevated ferritin > 500 (> 10000 = 90% sensitive and 96% specific for HLH)
- Low NK cell activity
- Elevated soluble CD25 (soluble IL2-R)
- Hemophagocytosis in BM, spleen, or LN: Only seen in later course of the diseases and not required for the diagnosis, neither sensitive nor specific, can be seen in severe sepsis/critical illness)
Management
- Like all things in medicine, treat the underlying cause
- Current treatment is based on the HLH-94 study on pediatric population
- Induction: 8 weeks dexamethasone and etoposide.
- Maintenance: Cyclosporine, tacrolimus, dex pulses
- If MAS: Steroids alone, usually responsive.
- Hematopoietic stem cell transplant is refractory/relapsing.
For more information on HLH, please refer to this article by Dr. Schram and Dr. Berliner published in Blood (as in the journal) in 2015.