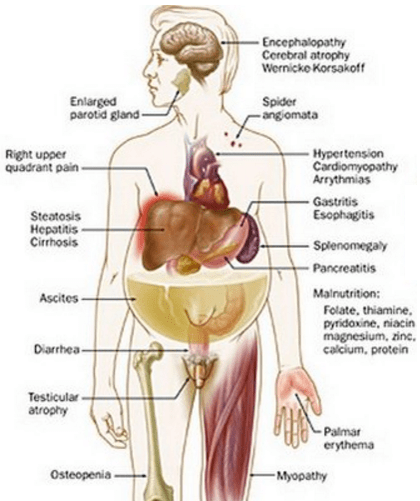
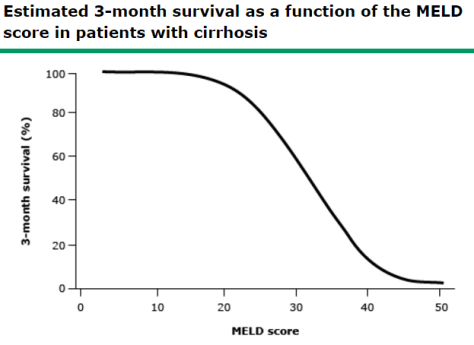
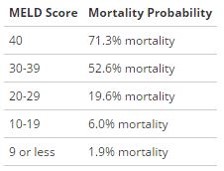
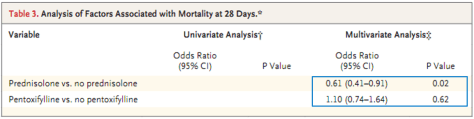
NuvaRing:
- Combined (progestin/estrogen) hormonal contraceptive vaginal ring
- Works by preventing ovulation and inhibition of sperm penetration (via cervical mucosal changes)
- Used for a 3 week period followed by a “break week;” reported 91% effective with typical use
Appendicitis:
Epidemiology: Most common indication for emergent abdominal surgery in childhood (<14 years old); Males > Females.
Pathophysiology: Non-specific obstruction of the appendiceal lumen (fecal material, undigested food, enlarged lymphoid follicle, etc.)
Clinical Manifestations: Anorexia, periumbilical pain (early) → migration to RLQ (often within 24 hours), vomiting (after onset of pain), fever (24-48 hours after symptoms)
Diagnosis: Clinical diagnosis; various scoring systems to aid treatment (PAS, Alvarado score, etc.), CBC (leukocytosis), +/- imaging (US vs. CT)
Treatment: Surgical resection

Pelvic Inflammatory Disease (PID):
Epidemiology: Sexually active females, younger age (15-25 yo), prior STIs, previous PID are all known risk factors; method of contraception also important (barrier is protective)
Pathophysiology: Two stages: Stage 1) acquisition of a vaginal or cervical infection (often STI); Stage 2) direct ascent of microorganism
Clinical Manifestations: Fever, nausea/vomiting, severe pelvic/abdominal pain, abnormal vaginal discharge (75% of cases), unanticipated vaginal bleeding, tenderness on pelvic exam (adnexal tenderness 95% sensitive)
Diagnosis: History/Physical, pregnancy test, CBC (leukocytosis), saline microscopy of vaginal fluid, ESR/CRP, STI testing, UA, +/- imaging
Treatment: Antibiotics against common organisms:
- Regimen A: ceftriaxone, doxycycline, metronidazole
- Regimen B: cefoxitin, doxycycline, metronidazole

Ectopic Pregnancy:
Epidemiology: Increased incidence (4.5/1000 pregnancies in 1970 vs. 19.7/1000 in 1992) attributed to improved diagnostics; more common in women > 35 years old and non-white ethnic groups.
Pathophysiology: Any pregnancy in which the fertilized ovum implants outside the intrauterine cavity (>95% in the fallopian tubes)
Clinical Manifestations: Abdominal pain with spotting ~ 6-8 weeks after the last menstrual period; physical findings include slightly enlarged uterus, pelvic pain with movement of the cervix, and palpable adnexal mass
Diagnosis: History/Physical, pregnancy test, +/- progesterone, US
Treatment: Depends on patient stability:
- Expectant management: 68-77% resolve without intervention
- Medical management: methotrexate
- Surgical resection: salpingectomy via laparotomy
Ovarian Torsion:
Epidemiology: 5th most common surgical emergency in females; primary risk factor is an ovarian mass (particularly >5cm) or pregnancy (20%), can occur at any age – but most common in early reproductive years (median age 28 years)
Pathophysiology: Ovary rotates around both the suspensory and utero-ovarian ligament; rotation results in compression of the ovarian vessels (vein before artery) leading to ovarian edema and eventually ischemia
Clinical Manifestations: Classic presentation: acute onset of moderate-severe pelvic pain (90%), often with nausea/vomiting (47-70%), in a women with an adnexal mass (86-95%); other symptoms include fever (2-20%) and abnormal vaginal bleeding (4%)
Diagnosis: History/Physical, pregnancy test, CBC, pelvic US with doppler
Treatment: Surgical evaluation with detorsion or resection
Yersina Enterocolitica:
Epidemiology: Most often due to consumption of raw or undercooked pork; young individuals more often (75% are 5-15 years old)
Pathophysiology: Following consumption, invasion and penetration occurs in the ileum (M cell) => multiplication in Peyer patches (underlying lymphoid tissue) => mesenteric lymph => node spread => localized infection => systemic infection (rare)
Clinical Manifestations: Fever, abdominal pain, and diarrhea ~ 4-6 days after exposure; pain often localized to the right-side of the abdomen (pseudoappendicitis)
Diagnosis: History/Physical, stool culture for yersinia
Treatment: Supportive care typicially; aminoglycosides and TMP-SMZ if severe
Pyelonephritis:
Definition:
- Pyelonephritis is an infection of the upper urinary tract, specifically the renal parenchyma and renal pelvis
- Cystitis refers to an infection of the lower urinary tract, specifically the bladder
Epidemiology:
- Women > Men (11.7 vs 2.4 hospitalizations per 10,000 cases)
- Men > Women (16.5 vs 7.3 deaths per 1000 cases)
|
PID |
Cystitis |
Pyelonephritis |
| Dysuria |
+ |
+ |
+ |
| Discharge |
+ |
|
|
| Abdominal Pain |
+ |
+ |
+ |
| Fever |
+ |
|
+ |
| Frequency/Urgency |
+ |
+ |
+ |
| CMT |
+ |
|
|
| CVA |
|
|
+ |
Pathogenesis:
- Most renal parenchymal infections result from bacterial ascent through the urethra and urinary bladder
- In males – prostatitis and prostate hypertrophy (causing urethral obstruction) predispose to bacteriuria
Uncomplicated Pyelonephritis:
- Typical pathogen
- Immunocompetent patient
- Normal urinary anatomy/renal function
Treatment:
Outpatient:
- Oral fluoroquinolone (i.e. ciprofloxacin)
- Recommended given low resistance rates (1-3%), absorbed well from the GI tract, excellent kidney penetration
- Other acceptable options for susceptible organisms include:
- Amoxicillin-clavulanate (preferred for pregnancy)
- Cephalosporin
- Trimethoprim-sulfamethoxazole
Inpatient:
- IDSA recommends one of three IV therapies:
- Fluoroquinolone (i.e. ciprofloxacin)
- Aminoglycoside (i.e gentamycin) +/- ampicillin
- Extended-spectrum cephalosporin +/- aminoglycoside
- 7-14 days is effective; but studies suggest that 5-7 is comparable to longer duration in terms of clinical and bacteriologic outcome
- Therapy with appropriate empiric antibiotics should produce improvement within 48-72 hours; failure should additional testing for an alternative diagnosis