Thanks to Amit for presenting the fascinating case of a middle-aged woman with history of DM2 who presented with subacute onset of unilateral periorbital pain, L CN 6 palsy, and L otorrhea, with MRI findings of petrous apicitis consistent with the super rare Gradenigo syndrome!
Clinical Pearls
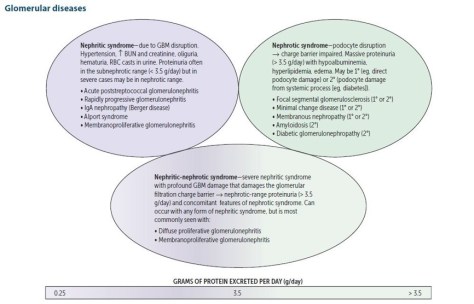
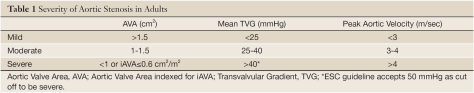
- Gradenigo syndrome is a rare and life threatening complication of otitis media and involves inflammation of the medial aspect of the temporal bone, specifically the apex of the petrous bone (a pyramid shaped bone jutting medially from the temporal bone)
- Gradenigo is clinically characterized by a triad of otorrhea, diplopia (due to CN6 palsy), and hemifacial pain (CN5 palsy).
- This is a very rare complication since most cases of otitis media are treated with antibiotics early on.
- Remember that a common cause of an isolated CN 6 palsy in a diabetic patient is diabetic neuropathy/ophthalmoplegia. A patient who has more cranial nerves affected than CN6 alone, you should be concerned about cavernous sinus thrombosis.
Gradenigo syndrome:
- First described in 1904 by Guiseppe Gradenigo.

Source: AO Surgery Reference - A rare and potentially life threatening complication of otitis media involving the inflammation of the apex of petrous pyramid (medial aspect of temporal bone). Occurs any time between 1 week to 3 months after acute otitis media (AOM) and up to 3 years after chronic suppurative otitis media (CSOM).
- Should suspect this syndrome any time there is CN 6 palsy in the setting of otitis media, whether acute or chronic
- Clinically, Gradenigo syndrome is characterized by triad of ear discharge, diplopia, and hemifacial pain
- Suppurative otitis media (ear discharge and pain)
- Trigeminal neuralgia involvement causes pain in the distribution of the nerve manifested as hemicranial headache and hemi-facial pain
- Abducens nerve involvement causes ipsilateral lateral rectus palsy and lateral gaze palsy
- Infection spread from suppurative otitis media to the petrous apex may be via pneumatized air cell tracts, through vascular channels, or as a result of direct extension through fascial planes
- Organisms are not well studied but the most common one appears to be pseudomonas. Staph, strep, pneumococcus, and TB have also been reported.
- If left untreated, it can result in serious complications such as meningitis, intra-cranial abscess, sinus thrombosis
- Treatment
- Broad spectrum antibiotics IV for up to 6 weeks (to treat a presumed temporal bone osteomyelitis)
- Fluoroquinolone ear drops
- Tight glucose control
- Differential diagnoses to consider:
- Cavernous sinus thrombosis
- Headache
-

Source: UpToDate Papilledema
- CN palsies (see picture of what runs through cavernous sinus)
- Ophthalmoplegic migraine:
- Rare condition, often manifests in children and young adults
- Diagnosis of exclusion
- Most commonly affects CN3 (but can go to CN4 and CN6 as well)
- Can sometimes precede the headache
- Review article here
- Diabetic ophthalmoplegia
- Common cause of isolated CN6 palsy
- Neoplasms
- Nasopharyngeal cancer
- Plasmacytoma
- Pituitary adenoma
- CN6 neuroma
- Skull base tumors
- Sohenoid sinus tumors
- Squamous cell
- Stroke
- Demyelinating diseases
- Vasculitis
- Idiopathic intracranial hypertension
- Cavernous sinus thrombosis
Complications of acute otitis media
- Intratemporal
- Tympanic membrane rupture (leads to hearing loss and pain relief!)
- Labrynthitis (nausea, vomiting, tinnitus, vertigo)
- Mastoiditis
- CN palsies (including Gradenigo syndrome)
- Extratemporal
- Epidural, subdural, and brain abscesses
- Skull base osteo
- Otitic hydrocephalus (without meningitis or brain abscess)
- Otitic meningitis
- Lateral sinus thrombosis